For decades, Chinese patients have struggled to gain access to cutting-edge medicines thanks to bureaucratic delays that have hamstrung drug development. Now a sweeping government overhaul of drug approvals is poised to change that.
Beijing on Sunday announced new rules that will speed up approvals of medicines and medical devices, easing bottlenecks in introducing new treatments. The move is also a growth opportunity for international and local drugmakers in the world’s second biggest pharmaceutical market. It also parallels the acceleration of approvals by the U.S. Food and Drug Administration.
Under China’s new rules, data from overseas clinical trials can be used for drug registrations in the country. That removes the need for manufacturers to conduct added tests in China after receiving overseas approvals and will likely cut delays in the launch of new drugs by several years.
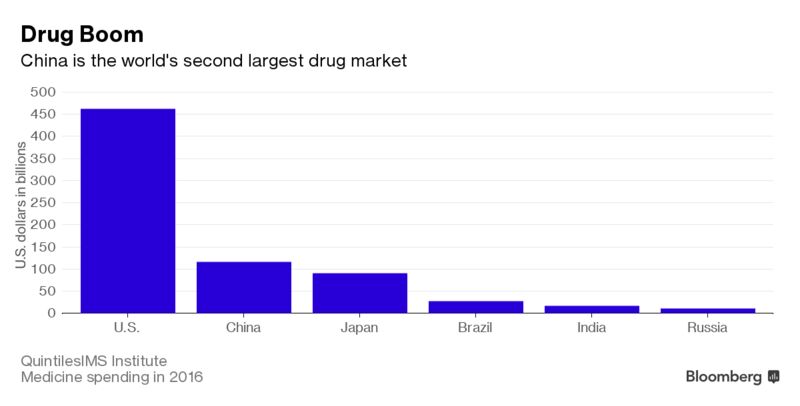
Faster approvals could deliver a revenue boost in coming years to Pfizer Inc., AstraZeneca Plc,GlaxoSmithKline Plc and other multinationals that are expanding there. China spent $116.7 billion on medicine in 2016 and the market is second only to the U.S. in size, according to researcher QuintilesIMS. China is revamping its drug regulatory system as demand for new therapies surges due to an aging population and rising incidence of diseases such as cancer and diabetes.
“For multinational and leading local innovative drugmakers, the anticipated acceleration of approval will improve patients’ access to new medicine and increase revenues for pharmaceutical companies,” said Jialin Zhang, senior health-care analyst at ICBC International Research Ltd. Foreign manufacturers control about a quarter of the Chinese pharma market, with the rest held by local players, he estimates.
The changes were announced by the State Council, China’s cabinet, just days before a key leadership gathering in Beijing next week. On Oct. 18, delegates will gather for the 19th National Congress of the Communist Party, a twice-in-a-decade shuffling of China’s political decks.
Promoting Innovation Due to insufficient innovation, the pharmaceutical and medical device products marketed in China fall short of international advanced standards, according to the State Council policy statement. The reforms aim to promote restructuring and innovation in order to meet the public’s clinical needs, it said.
In the short term, foreign drugmakers might be the prime beneficiaries because they’re already starting to see quicker approvals for their drugs and have deep pipelines of medicines in development, Zhang said. Most local drug companies are still climbing the innovation ladder. That said, Chinese rivals might be bigger beneficiaries over the long-term thanks to expertise in the local market and cheaper costs, he said.
For now, most international pharmaceutical companies get only a small fraction of their global sales from China. But they still count on the country to serve as a growth driver given its vast unmet medical needs and a burgeoning middle class that can increasingly afford cutting-edge treatments.
Local and multinational drugmakers have for years struggled with delayed approvals in China as a surging number of applications and a relatively small team of government reviewers resulted in a regulatory backlog. The delays in access to life-saving therapies led Chinese patients to buy drugs from grey markets over the Internet or from bootleggers, putting them at risk of receiving counterfeit drugs.
Both Pfizer and AstraZeneca said they welcome the new rules.
“China is a key growth market for AstraZeneca and we are working closely with authorities to ensure our medicines are accessible by the many patients who can benefit from them,” the Cambridge, England-based company said in a statement.
The policies will “pave the way for China’s integration into the system for multiregional clinical trials that supports global drug development,” New York-based Pfizer said in its own statement.
Bold Reforms The changes announced Sunday had already been widely telegraphed by the Chinese government, which earlier this year said it was considering overhauling the approval process. The China Food and Drug Administration has been introducing bold reforms in recent years, and the latest policy appears to have received the blessing of top-levels of the central government, said Zhang.
More recently, the China FDA has already been working to reduce the backlog. That has already led to speedier approvals for some treatments like AstraZeneca’s lung cancer therapy called Tagrisso, estimated to become a global blockbuster next year. As more innovative drugs make an entrance, foreign manufacturers will still have to manage rising price pressures in China. The government has sought to cut prices to manage costs in its public health insurance system, putting foreign drugmakers through more negotiations with hospitals and local governments and squeezing margins.
Patent Protection The reforms announced Sunday include other measures to speed up approvals for clinically needed drugs and equipment, establish a compulsory-licensing system and make it easier for research institutions to conduct clinical trials, according to the document.
The government said it will also explore a new system linking drug approvals to patent status. This could potentially delay the introduction of generics when there are legal challenges posed by the patent holder.
China’s protection of intellectual properties is still lacking, “and this is also an important reason that restricts the development of our medical innovation industry,” said Wu Zhen, vice minister of China FDA, at a press briefing webcast on Monday.
Measures on patents were previously adopted by developed markets such as the U.S., Europe and Japan, and their implementation helped boost both innovative companies and generic drugmakers, Wu said.
In the U.S., the FDA is taking advantage of policy groundwork laid in past years to speed drug approvals. Thirty-four new drugs have been approved so far this year -- on pace to nearly double from last year.
— With assistance by Hui Li
 |手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033
|手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033