金币
UID13624
帖子
主题
积分8624
注册时间2012-1-17
最后登录1970-1-1
听众
性别保密
|
欢迎您注册蒲公英
您需要 登录 才可以下载或查看,没有帐号?立即注册
x
Challenging The Streamlined Approach
具有挑战新的简化方法
The approach recommended above is a simplified process compared to what youwould normally undertake for specifying a major computer system, and as such normally generates a number of questions. The following questions and answers pre-emptthose questions and give the authors view on why the approach is ideally suited forthe applications we are dealing with.
上面建议的方法和你通常会指定一种主要的计算机系统相比而言是一种简化的方法,这种方法通常会产生一系列的问题。下面的问题和回答会涉及到这些问题并且给出作者在为什么该方法理想地适应我们要处理的应用程序上的观点。
1. Question. Is there any regulatory document that states you can/cannotcombine the
URS with the FS?
问题:有没有任何法规文件规定你可以/不可以讲 URS 和 FS 合并
No, not that we are aware of! Companies will be expected to document User Requirements, and how the system functions. How you document it is not normally defined, only that it is clear, accurate and allows traceability.
不,就我们所知没有。公司将会被期望归档用户需求以及系统运作。你如何归档它并没有一个通用的规定,仅仅保证它清晰、准确和有可追溯性就可以了。
2. Question. Our QA policy states that we must have a separate URS!
问题:我们的 QA 制度规定我们必须有一份独立的 URS
The recommended approach is an attempt to save you time and resources by streamlining the process. If your policies state that you must work in acertain way then unfortunately you will have to follow them. Being out of compliance with yourown procedures is a serious breach, and one which is difficult to defend.
推荐的方法只是通过简化的程序帮助你节省时间和资源。如果你们的制度规定你必须在特定的方法下工作,那么你必须遵从他们。不遵从你们自己的规程是一种非常严重的违约行为,并且是一种难以辩护的行为。
The simple answer to this dilemma is that you should modify your corporatepolicies and procedures to allow such a streamlined approach where appropriate. Mostcompanies allow this approach, and it is key in the new “risk based” validationphilosophies that people take.
对于这种困境的简单处理方法是你可以修改你们公司的制度和规程以在合适的地方允许这种合适的简化方法。大多数公司允许这种方法,并且关键是基于风险的验证观点。
Updating and improving your corporate policies in this way will benefit otherprojects in the future.
通过这种途径升版和改进你们公司的制度将有益于将来的其他项目。
3. Question. Normally the User generates the URS and the software supplergenerates the FS. How does this fit when we do it all ourselves?
问题:一般由用户生成 URS,软件供应商生成FS,当我们全部由我们自己做时怎么办?
The concept of user and supplier does not fit well for spreadsheets, as it isoften the user who developed the spreadsheet. A division between user needs and developingnever occurred as the development was undertaken as a prototyping activity.
这种用户和供应商的观点并怎么适合电子表格,因为电子表格通常由用户开发。用户需求和开发从来不以进行原型活动的开发来区分
Most spreadsheets are developed using a rapid application development (RAD),using prototypes and trial and error until it is ready to use. This approach iscommon and acceptable as long as you build your validation process around it.
大多数电子表格使用快速应用程式开发工具进行开发,使用原型、试验和错误直到可以使用。这种方法是常见的并且是可以接受的,只要你为围绕它建立你的验证程序。
When you prototype in this way the common practice is to retrospectivelygenerate your URS and FS. Retrospectively generating the FS is simple and makes perfectsense, whilst retrospectively generating a URS often seems strange as you list whatyou have, rather than what you need.
当你通过这种途径建立原型时,常见的做法是回顾性地生成你的 URS 和 FS。回顾性的生成 FS 是简单并且是十分有意义的,同时回顾性地生成 URS 经常看起来比较奇怪,因为你列出了你有的而不是你需要的。
Your auditor wants to see user needs and the actual operation specified, howyou get to this situation is secondary.
审计员希望看到的是用户需求和具体的实际操作,你是如何达到这种情况反而是次要的。
4. Question. Can we generate separate specification documents and still followthe approach being recommended?
问题:我们可以生成单独的规范文件并仍然遵从你推荐的方法吗?
Of course. Returning to the traditional approach of having separate documentsfor URS and FS is common, but will take additional work, and therefore cost you more inmoney and resources. It will also slow down the approval and review process.Experience has showed that the document review and approval process is often a bottle neck inthe validation process.
当然。回到有单独的 URS 和 FS 文件这种传统的方法是很常见的,但是这将带来额外的工作并且需要花费你更多的金钱和资源。它也会降低批准和审核的流程。经验表明,文件的审核和批准流程是验证程序的瓶颈。
5. Question. The headings recommended don’t match our corporate procedures on what should exist in a specification document.
问题:标题部分并不符合我公司关于规范文件应有什么的规程规定。
The headings provided are the generic ones and often companies wish to changethem to match up with their own corporate standards which is fine. These headings areprovided to ensure you cover the key subjects and items that need to be specified.
标题中的信息只是通用的,各公司经常变更他们以更好的适应各自的标准。标题的这些内容只是确保你涵盖了需要制定的关键主题和项目。
6. Question. A generic specification implies that the content is the same formany of our spreadsheets. All our spreadsheets are very different.
问题:通用的规范意味着我们许多电子表格内容都是相同的。但实际上我们电子表格有着很大的区别
Every spreadsheet is different, however many of the underlying requirementsfrom a spreadsheet are the same or very similar.
每一个电子表格都是不同的,然而电子表格许多基础的需求都是一样的或者是相似的。
For example,
• Your need to have procedures and documentation in place, such as spreadsheet operation, backup and restore and training records does not change.
• Your need to comply with 21 CFR Part 11 by having logical security and anaudit trail does not change, and therefore your specification would be worded the same foreach spreadsheet.
比如:
l你需要在适当的位置有规程和文件材料,比如电子表格操作、备份和恢复以及培训记录,这些并不会变
l你需要有逻辑安全措施和审计追踪功能符合 21 CFR Part 11,这是不变的。并且因此对于每一份电子表格,你的规范措辞都一致
Clearly the evidence that each of these requirements has been met would need tobe verified individually at the qualification stage, but at the specificationstage many of these needs and responses are identical.
毫无疑问的是,每一条需求都应符合的证据都需要在确认阶段单独进行确认。但是在规范阶段许多这些需求和响应都是相同的。
Certain sections of the specification (such as Appendix A, B, and C) will bevery different for each spreadsheet. This is the reason these sections are taken out intoAppendices; it allows the document writer to know immediately the areas that need closeattention in the generation process.
规范的某些部分(如附件 A、B 和 C)对于每一个电子表格都是不同的。这就是为什么把这些章节作为附件。这将允许文件起草者立即知道在规范生成流程中哪些区域是需要密切关注的。
7. Question. If we use macros do we have to printout the source code in the specification?
问题:如果我们使用宏,那么我们必须在规范中将源代码打印出来吗?
There is no specific need to list the macro’s source code, but with spreadsheetmacros it is usually done this way for completeness due to the fact that the code listingis small, and the effort and confidentiality issues over printing are minor.
理出宏的源代码并不是特定的需要。但是有宏的电子表格为了完整性经常这样做,因为事实上代码清单很小,并且这样做和保密性问题反而是次要的。
8. Question. Our spreadsheet is huge, do we have to printout all the formulas?
问题:我们的电子表格很大,我们需要将所有公式都打印出来吗?
Normally the whole content of the spreadsheet would be printed out and storedin Appendix B. However, on very large repeated spreadsheets this is oftenoverkill. Many spreadsheets will have the same formulas repeated in an identical fashion formany hundreds or thousands of rows, often the only thing that changes is the rowreference in the formula. In this situation we would list the content to shorten thespecification content (see Table. 4).
一般来说,电子表格的整个内容在附件 B 中打印和保存。然而,对一些非常大的重复的电子表格,这显得有些过分了。许多电子表格在成百上千的行中有着相同的重复公式,唯一变化的只是公式中的参考行。这种情况下,我们会列出如表 4 所示的内容以缩短整个规范的长度。
Table. 4 Example of procedure for repeated large spreadsheet
表 4:大量重复的电子表格程序示例
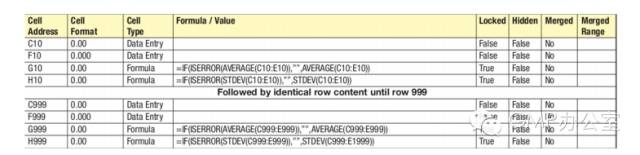
This presents minimal risk, and with recent error checking tools in Excel4, ifindividual rows were inconsistent you would see the error at the prototyping or qualificationstage.
这会有极小的风险,通过最新的 Excel 错误检查工具,如果个别行出现不一致,你将会在原型或确认阶段看到错误。
9. Question. Our spreadsheet is huge, do we have to printout all the formulasto paper?
问题:我们的电子表格是巨大的,我们是否需要将所有的公式在纸上打印出来?
Normally this would be done, but sometimes electronic media can be used to help save on paper. Our preferred option would be to run our reporting tools on thespreadsheet and convert the generated table into PDF format and burn it onto CD ROM. This wouldthen stored in a wallet in Appendix B.
一般来说需要这么做,但是有些时候可以使用电子介质来代替纸质储存。我们优先的选择是在我们的电子表格上运行报告工具并将生成的表格转换成 PDF 格式并烧录在CD-ROM 中。然后作为一个包保存在附件 B 中。
10. Question. Our recent inspection/auditor stated that a separate URS wasneeded for System X? We can’t risk combining the URS or Our recent auditor stated thatsource code must be printed for custom programming!
问题:我们最近的检查/审计员规定系统 X需要一份单独的 URS?我们不能冒险整合URS 或者我们最近的审计员规定必须打印自定义编程的源代码。
Individual auditors and inspectors will always have their own opinion on manyof the points discussed above. The lack of hard and fast rules makes computer systems validation a difficult area to interpret. In this situation you can only dowhat you think iscorrect and defend your approach.
个别的审计员和检查员在上面讨论的问题总是有他们自己的观点。缺乏硬性的规定使得计算机系统验证是一个难以解释的区域。这种情况下,你只能按照你自己认为正确的做并坚持你的方法。
Be careful not to confuse what an auditor asked for with your complex systemswith what they will expect with a spreadsheet or standalone instrument, they are notcomparable and neither should your validation approach be.
注意不要将审计员针对你复杂的系统的要求和他们对电子表格或独立的仪器期望弄混淆,他们没有可比性并且你的验证方法同样也没有。
The approach presented in this paper has been through many inspections, and todate it has not been challenged by a regulatory inspector. It has however beenchallenged on many occasions for not meeting local QA requirements, or for being different tothe validation approach taken for other more complex systems.
本文所提出的方法已经通过了许多检查,并且迄今为止还没有受到法规部门检查员的挑战。它只是在许多场合不符合当地 QA 的要求,在作为其他更多的复杂系统的验证方法时,也有很大的不同。
There is a simple (but not recommended) answer to this dilemma, and thatis to perform the full validation lifecycle on each individual spreadsheet. Generating a VP,URS, SDS, Source Code Review, FS, IQ, IQR, OQ, OQR, PQ, PQR, VR for each spreadsheetwould certainly solve your worries over consistency and completeness. It wouldhowever also financially cripple your validation effort and tie up valuable resources inareas which most people would consider unnecessary. You could also ask the question as towhether an extensive ‘full lifecycle’ suite of documents is any more likely to ensure an error-free spreadsheet? Our belief is that it does nothing to reduce spreadsheet errors,but is far more likely to introduce documentation errors.
这是一个对这种困境的简单的(但是不推荐)回答:对每个单独的电子表格在其整个验证生命周期里进行。每一份电子表格都生成一份验证计划(VP),用户需求标准(URS),软件设计标准(SDS),源代码审核、功能标准(FS)、安装确认(IQ)、安装确认报告(IQR)、运行确认(OQ)、运行确认报告(OQR)、性能确认(PQ)、性能确认报告(PQR)、验证报告(VR)当然可以解决你在一致性和完整性方面的担忧,但它会在财政上削弱你的验证工作并且占用大量宝贵的资源,这也是大多数人认为是不必要的。你同样可以问这样一个问题,一套广泛的完整生命周期文件是否有误可能更加确保一份电子表格无错?我们相信它并不会降低电子表格出错的可能,反而更可能带来文档错误。
The streamlined and pragmatic approachdescribed in this article is about taking measured risks, and presenting thecritical information in a clear yet easy to generate format.
本文所描述的简化的和实用的方法即考虑到衡量风险并以清晰且易生成的方式给出了关键信息。
Conclusion
总结
Astreamlined approach to the specification of Excel spreadsheets has beenoutlined in thisarticle. This approach condenses best industry practices into one generic specificationdocument. This approach offers a pragmatic way to undertake a cost effectiveand repeatable process and it can be adapted to simple systems other than spreadsheets.
本文概述了一种简化的 Excel 电子表格规范的方法。这种方法将最好的工业实践凝聚成一份通用的规范文档。这种方法提供了一个实用的途径以进行效率成本和可重复的流程,并且除了电子表格还适合用于简单系统。
|
|
 |手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033
|手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033