欢迎您注册蒲公英
您需要 登录 才可以下载或查看,没有帐号?立即注册
x
WHO 第992号技术报告 附录3 非无菌工艺验证 1. Background and scope 背景和范围 WHO第937号技术报告中公布了GMP增补指南:验证,此外还公布了一些指南来支持现行的GMP实施方法。这些指南意在为WHO和ICH的质量源于设计原则(QbD)和质量风险管理(QRM)提供更多的工艺验证概念支持。 这些指南允许使用不同的方法来实现工艺验证。其所描述的原则主要适用于非无菌制剂。类似方法也可以用于原料药和无菌产品。(参见WHO第957号技术报告附录2,以及WHO第961号技术报告附录6)。推荐采用基于风险的生命周期方法来进行验证。 由于现在主要聚焦于生命周期方法,因此在所有工艺验证方法中均需要对产品和工艺研发研究的深入的知识、之前的生产经验以及应用QRM原则。 生命周期方法将产品和工艺研发、商业化生产工艺的验证、在日常商业化生产中将生产工艺维持在受控状态结合了起来。 推荐使用过程分析技术(PAT),包括远线、近线和/或在线控制和监测技术,以保证工艺在生产过程中处于受控状态。 2. Glossary 术语(略) 3. Introduction 概述 Process validation data should be generated for all products to demonstrate the adequacy of the manufacturing process. The validation should be carried out in accordance with GMP and data should be held at the manufacturing location whenever possible and should be available for inspection. 所有产品均应生成工艺验证数据,以证明生产工艺的充分性。所实施的验证应符合GMP要求,验证数据应保留在生产场所,检查中应可以获取。 Process validation is associated with the collection and evaluation of data throughout the life cycle of a product – from the process design stage through to commercial production – and provides scientific evidence that a process is capable of consistently delivering a quality product. 工艺验证与产品从工艺设计阶段到商业化生产的整个生命周期中数据收集和评估过程紧密相关,它提供科学证据证明一个工艺可以持续地产生一个具备所需质量的产品。 A risk assessment approach should be followed to determine the scope and extent to which process(es) and starting material variability may affect product quality. The critical steps and critical process parameters should be identified, justified and documented and based on relevant studies carried out during the design stage and on process knowledge, according to the stages of the product life cycle. During process validation and qualification, the critical process parameters should be monitored. 应采用风险评估方法来确定工艺的范围和深度,以及可能影响产品质量的起始物料波动。应根据设计阶段所实施的相关研究和对工艺的理解,根据产品生命周期的不同阶段,对关键步骤和关键工艺参数进行识别、论述和记录,在工艺验证和确认期间,应对关键工艺参数进行监测。 It may be helpful to use a flow diagram depicting all the operations and controls in the process to be validated. When applying QRM to a given operation, the steps preceding and following that operation should also be considered. 绘制要验证的工艺中所有操作和控制流程图将有所帮助。在对一个给定的操作实施QRM时,也应考虑该操作前后的步骤。 Amendments to the flow diagram may be made where appropriate, and should be recorded as part of the validation documentation. 适当时应对流程图进行修正,修正内容应记录作为验证文件的一部分。 Manufacturers should ensure that the principles of process validation described in these guidelines are implemented. These cover the phases of validation during process design, scale-up, qualification of premises, utilities and equipment and process performance qualification, and continuous process verification to ensure that the process remains in a state of control. 生产商应保证按本指南中所述的工艺验证的原则实施工艺验证。这包括了在工艺设计、放大、厂房确认、公用系统和设备和工艺性能确认、持续工艺确认中的各验证阶段,以保证工艺保持在受控状态。 The objectives of process validation include ensuring that: 工艺验证的目的包括保证: - the process design is evaluated to show that the process is reproducible, reliable and robust; - 工艺设计经过评估,显示出工艺具可重复性、可靠性和耐用性 - the commercial manufacturing process is defined, monitored and controlled; - 对商业生产工艺进行了定义、监测和控制 - assurance is gained on a continuous basis to show that the process remains in a state of control. - 保证工艺持续保持在受控状态 The validation should cover all manufactured strengths of a product and the extent of validation at each manufacturing site should be based on risk assessment. A matrix approach or bracketing may be acceptable and should also be based on appropriate risk assessment. 验证应包括生产产品的所有剂量,各生产场所的验证深度应根据风险评估来确定。可以使用矩阵法或括号法(分组法),这些方法的使用也应该是基于适当的风险评估。 There are various approaches to process validation which include: traditional process validation (consisting of prospective and concurrent validation); process design followed by process qualification and continued process verification; or a combination of traditional process validation and the new approach described in these guidelines. Historical data should be evaluated in cases where there have been changes to the process. 可以有不同的工艺验证方法,包括:传统工艺验证(包括前验证和同步验证)、工艺设计和之后的工艺确认和持续工艺确认,或传统工艺验证方法与这些指南中所述的新方法的结合。如果对工艺进行了变更,则需要对历史数据进行评估。 Manufacturers should plan to implement the new approach to process validation, which covers process design, process qualification and continued process verification throughout the product life cycle. 生产商应计划实施新的工艺验证方法,它包括工艺设计、工艺确认和持续工艺确认,贯穿产品的整个生命周期。 Figure A3.1 shows the phases in the new approach to process validation. 图A3.1展示了新的工艺验证方法的各阶段。 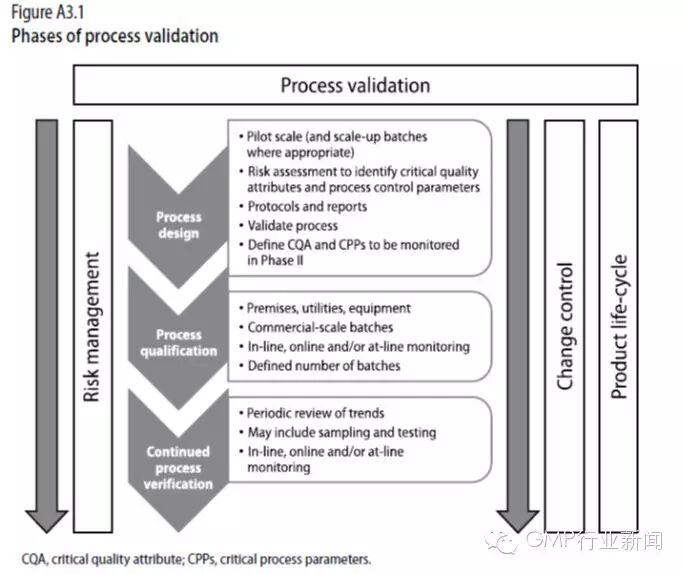
|  |手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033
|手机版|蒲公英|ouryao|蒲公英
( 京ICP备14042168号-1 ) 增值电信业务经营许可证编号:京B2-20243455 互联网药品信息服务资格证书编号:(京)-非经营性-2024-0033